|
|
Common Meteorological Variables
|
|
|
|
|
|
|
Principle
|
Air is a compressible mixture of gases.
It has properties such as temperature, pressure and density.
It obeys the laws of thermodynamics and fluid mechanics,
including the ideal gas law.
|
|
|
|
|
|
|
Table 1 - Composition of the Atmosphere
|
|
Gas
|
% by
Volume*
|
|
Nitrogen
|
~78%
|
|
Oxygen
|
~21%
|
|
Water Vapor
|
~0 to 4%
|
|
Argon
|
~0.93%
|
|
Carbon Dioxide
|
~0.035%
|
|
Neon
|
~0.0018
|
|
Helium
|
~0.0005%
|
|
Methane
|
~0.00017%
|
|
Hydrogen
|
~0.00005%
|
|
Nitrous Oxide
|
~0.00003%
|
|
Xenon
|
~0.000009%
|
|
Ozone
|
~0.000004%
|
|
Particles (dust, salt, pollens, etc.)
|
~0.000001%
|
|
Chlorofluorocarbons (CFCs)
|
~0.00000001%
|
* Does not add to 100% because of the
variability of water vapor.
|
|
|
|
|
|
|
|
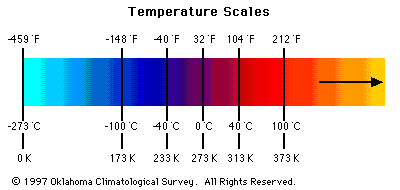
Figure 1 - Temperature Scales
|
|
|
|
|
|
Temperature
|
- The coldest temperature measured on Earth was
-127 deg F (Vostok, Antarctica).
|
|
|
|
|
|
|
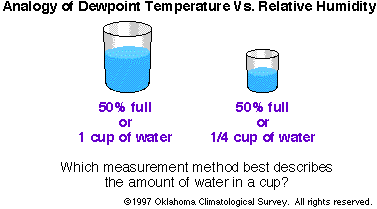
Figure 2- Dewpoint Temperature and Relative Humidity
|
|
|
|
|
|
Dewpoint Temperature and Relative Humidity
|
- Dew point (or dewpoint
temperature) is a measure of atmospheric moisture.
The dew point is the temperature to which air must be
cooled for saturation to occur.
The best way to understand dew
point (and the existence of water vapor in the air) is to
take a cold glass of ice water outside on a warm day and
watch the beads of water condense on the outside of the
glass. When the glass cools the air to its dewpoint
temperature, the moisture in the air condenses on the
outside of the glass.
- Relative humidity is the ratio
of the amount of water vapor actually in the air compared
to the maximum amount of water vapor the air can hold at
that particular temperature and pressure.
- Dewpoint temperature is a
better measure of moisture in the air than is relative
humidity.
|
|
|
|
|
|
|
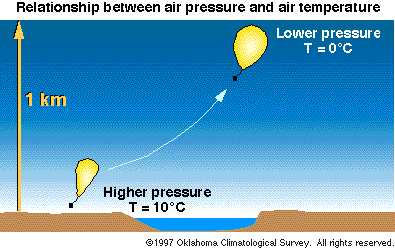
Figure 3- Relationship Between Air Pressure and Air
Temperature
|
|
|
|
|
|
Air Pressure
|
- As a gas, air obeys the ideal gas law. Hence, when
the pressure of a given volume of air (called an air
parcel) decreases, the volume (or parcel) cools.
Likewise, when the pressure of an air parcel increases,
the parcel warms.
As a result, an ascending air
parcel always cools. A descending air parcel always
warms. (Pressure always decreases with height in the
atmosphere.)
- The rate at which the parcel cools or warms as it
ascends or descends is dependent on the moisture content
of the parcel.
An air parcel with no water
vapor in it will cool or
warm at a rate of is about 10 degrees Celsius for every
kilometer change in altitude (or 5.4 deg F per 1000
feet). A moist,
unsaturated air parcel
(e.g., relative humidity below 100%) will cool or warm at
the same 10 deg C per kilometer. A saturated air parcel (e.g., condensation has occurred) cools
or warms at a rate generally between 4 deg C and 7 deg C
per kilometer (2 deg F to 3.5 deg F per 1000 feet).
(These "lapse rates" are different because condensation
adds heat to the air and evaporation removes heat from
the air.)
|
|
|
|
|
|
|
Wind, Air Density, and Water Vapor Mixing Ratio
|
- Wind is simply the velocity of the air with respect
to the earth.
In meteorology, we refer to the
wind direction as the direction from which the wind is
blowing. Thus, a north wind is air blowing
from the north toward
the south.
- Wind results from physical
forces acting on the air.
These forces result from
gravity, buoyancy, friction, differences in pressure
(pressure gradients), the rotation of the earth, and
centrifugal force, to name a few.
- The density of air at 0 deg
Celsius at sea level is 1.3 kilograms per cubic
meter.
- Warm air is less dense than
cold air; hence, warm air tends to rise.
- Moist air is less dense than
dry air; hence, moist air tends to rise.
|
|
|
|
|
|
|
End
|
|


